This is a prospective, randomized, parallel group, double-blind, sham-controlled, multicenter clinical trial to assess the safety and effectiveness of Bronchial Rheoplasty for the treatment of the symptoms of chronic bronchitis in moderate to severe COPD.
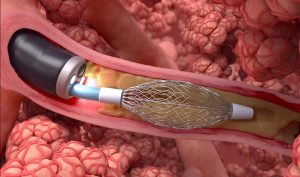
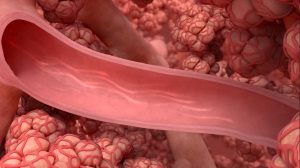
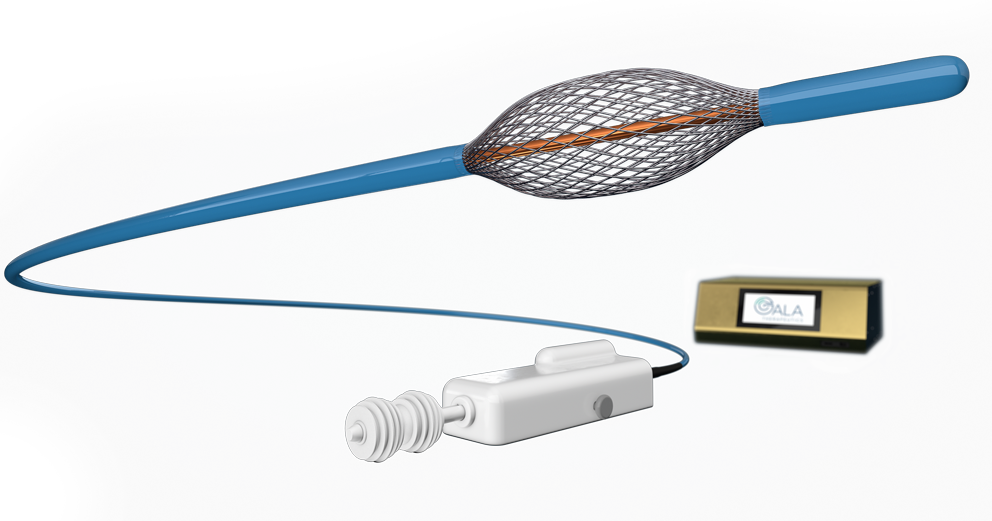
Bronchial rheoplasty is a staged procedure wherein pulsed electric fields are delivered to the goblet cells in the airways of right lung in the first treatment visit, and then the left lung is treated approximately 1 month later.
End Points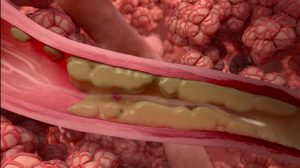
For more detailed information on this trial please click the link above to visit ClinicalTrials.gov